Why Drug Names Matter

By Alasdair Wilkins
On June 27, the FDA made its final decision on whether to approve Arena’s weight management drug “Lorqess;” later that day, it announced the approval of the drug, but with a new name: “Belviq.” Less than a month later, the FDA again had to decide whether to approve another weight management medication, this time Vivus’ Qnexa. Once again, the drug was approved, but under the new name Qsymia. The drugs had not been altered in any other way, and yet the FDA was only willing to give its stamp of approval to “Belviq” and “Qsymia,” not “Lorqess” and “Qnexa.” This begs the obvious question – “Why do some drug names prove acceptable, while others do not?” It’s a query that cuts to crucial issues about the relationship between patients, drug companies, and regulatory agencies. Indeed, while drug names might seem trivial, the core issue that concerns the FDA is one of safety. Companies fundamentally choose drug names for marketing and informational purposes, though the names that get approved by the FDA are those that are least likely to place patients in danger.
At first glance, that might seem extreme. After all, how much damage can a drug’s name really do? Well, when it’s the wrong drug name, the risk is substantial. The FDA’s Center for Drug Evaluation and Research (CDER) received 126,000 instances of medication errors between 2000 and 2009, many of which can be directly attributed to confusion between pairs of drug names that either sound or look alike. There are a number of reasons why such mistakes can creep into the process of prescribing medication. Let’s analyze just how drugs get their names, why errors occur, and what you need to know to ensure you get the right medication the next time you visit the pharmacy.
A Matter of Safety
First, prescriptions are often still handwritten as opposed to typed out, and some healthcare professionals don't always or ever take care to write neatly. Pharmacists might then have trouble deciphering the precise prescription, and even if the doctor or nurse's handwriting is legible, it’s still possible to mix up any two of the hundreds of FDA-approved drugs. And once a mistake has been made, a patient might not catch it straight away. For someone newly diagnosed with type 2 diabetes, it might not be immediately obvious that Amaryl is a trade name for the sulfonylurea glimepiride, a treatment for type 2 diabetes often used early in disease management (albeit one now used much less often in the United States), whereas Janssen’s Reminyl is used to treat dementia associated with Alzheimer’s disease. Indeed, according to Janssen subsidiary Ortho-McNeil, two deaths were reported in the early 2000s in cases where Amaryl was accidentally given to Alzheimer’s patients, which led to severe hypoglycemia. This prompted the FDA to take the highly unusual step of changing a drug’s name long after its approval.. Reminyl is now called Razadyne.
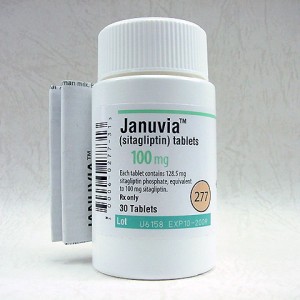 The Institute for Safe Medication Practices (ISMP) maintains an ongoing List of Confused Drug Names, which records hundreds of pairs of lookalike and soundalike drug names that have led to real, reported mix-ups. For instance, Merck’s DPP-4 inhibitor Januvia, designed for type 2 diabetes, appears three times on the ISMP’s list. One source of confusion is at least a close relative of Januvia – its own combined formulation with metformin, Janumet. This may sound like it wouldn’t hurt many patients who take it by accident (Janumet is just Januvia with the generic metformin added) but some patients cannot take metformin if they have certain medical problems such as kidney disorders, lung disease, or liver disease, all of which increase the risk of lactic acidosisBut Januvia has also reportedly been confused with Duramed Pharmaceuticals’ Enjuvia, which is a synthetic estrogen meant to treat the symptoms of menopause. Januvia has also been mixed up with Upsher-Smith’s Jantoven, a blood thinner also known and sold by its generic name warfarin. Beyond the obviously serious risks of taking Jantoven when one’s diabetes management depends on regular administration of Januvia, there’s another potentially major risk that could be caused by a mix-up – while Januvia is rated as a Category B drug, meaning it’s safe to take while pregnant, Jantoven is a Category X, meaning it should not be taken during pregnancy under any circumstances.
The Institute for Safe Medication Practices (ISMP) maintains an ongoing List of Confused Drug Names, which records hundreds of pairs of lookalike and soundalike drug names that have led to real, reported mix-ups. For instance, Merck’s DPP-4 inhibitor Januvia, designed for type 2 diabetes, appears three times on the ISMP’s list. One source of confusion is at least a close relative of Januvia – its own combined formulation with metformin, Janumet. This may sound like it wouldn’t hurt many patients who take it by accident (Janumet is just Januvia with the generic metformin added) but some patients cannot take metformin if they have certain medical problems such as kidney disorders, lung disease, or liver disease, all of which increase the risk of lactic acidosisBut Januvia has also reportedly been confused with Duramed Pharmaceuticals’ Enjuvia, which is a synthetic estrogen meant to treat the symptoms of menopause. Januvia has also been mixed up with Upsher-Smith’s Jantoven, a blood thinner also known and sold by its generic name warfarin. Beyond the obviously serious risks of taking Jantoven when one’s diabetes management depends on regular administration of Januvia, there’s another potentially major risk that could be caused by a mix-up – while Januvia is rated as a Category B drug, meaning it’s safe to take while pregnant, Jantoven is a Category X, meaning it should not be taken during pregnancy under any circumstances.
In a 2004 issue of its newsletter Medical Safety Alert, the ISMP published a story that serves as a harrowing example of the dangers of mixing up drug names. The ISMP reports that a person recently diagnosed with type 2 diabetes – and so potentially less likely to recognize a prescription error – was prescribed Takeda’s Actos at 30 mg daily. When the patient went to get the prescription filled, the pharmacist typed “ACTO” into the computer and clicked on the drug name that appeared on the selection screen, assuming it was Actos. However, the dispensed drug was actually Actonel, Warner Chilcott’s osteoporosis medication. It took over two full weeks before the error was noticed, and by that time the patient’s blood glucose levels had skyrocketed to 400 mg/dl. Of course, some people with diabetes do take months or even years to be diagnosed, so high numbers like that are far from uncommon – still, this was extremely unwelcome news for the patient, who likely thought the medication was getting his or her blood glucose under control.
Of course, all of these are instances of similar, confusable drug names that the FDA approved. The fact is that there’s no way to prevent such mix-ups entirely, which is why it’s so important to be vigilant whenever getting your prescriptions filled. Simply double-checking that you can read your physician or nurse’s writing is a great first step, and you can ask them to write down not just the name of the drug, but also its intended purpose – even something as basic as “for diabetes” on the prescription pad can help prevent the most serious mix-ups.
Knowing both the trade name and the generic name of your medication is also really smart – while Januvia, Jantoven, and Enjuvia could all be mixed up, it’s a lot harder to confuse sitagliptin, warfarin, and synthetic conjugated estrogens. If you’re uncertain whether your pharmacist has supplied you with the correct medication, ask him or her what the medication is meant to treat: if they start talking about anticoagulation and osteoporosis, then a mistake has probably been made. You can find a list of some potentially helpful tips and questions to ask here, and if you’re still uncertain you can always research the medication name online to double-check it does what it’s supposed to.
How Drugs Get Their Names
While it’s always good to know what you can do to avoid mistakes at the end of the prescription process, a pair of regulatory agencies do all they can at the beginning of this process to minimize mistakes as much as possible. Every approved medication in the United States has two names: its trade name and its generic name. Most diabetes drugs are still sold under their trade names – Byetta instead of exenatide, Lantus instead of insulin glargine, Victoza instead of liraglutide, and so forth – although older medications that have gone off-patent are now freely sold by multiple companies using their generic name. That’s why we talk about metformin instead of Glucophage, Glumetza, Fortamet, or Riomet. The FDA determines the trade names by which a drug is known for roughly its first twenty years of use, while the United States Adopted Name Council (USANC) works with international organizations to determine its generic name, which beyond its use once the drug goes off-patent is also the name used for medical research and educational materials.
The FDA’s Center for Drug Evaluation and Research is responsible for vetting a drug’s proposed name, with the safety review carried out by its Division of Medication Error Prevention and Analysis (DMEPA). When reviewing a new medication, DMEPA generates a long list of similar names that could conceivably be confused with the drug’s proposed name. The spelling, spoken pronunciation, and visual appearance of the word are all evaluated and compared with other existing trade names, as well as those of heavily used generic names like metformin. They also test the proposed name using a variety of handwriting samples of varying degrees of legibility. This can identify potential sources of confusion that might never be suspected otherwise. For instance, we generally don’t think of the letters “A” and “C” looking all that similar, but if they’re next to the right letters and the physician’s cursive handwriting is bad enough, they actually can be confused for one another. That might sound unlikely to you, but the ISMP lists two drugs that were confused with the now-withdrawn drug Avandia. One of these is Prandin, likely a result of the shared “-andi-” in the middle of each. The other, however, is Coumadin. The fact that Avandia and Coumadin don’t really seem to resemble each other at all speaks to how difficult this process is.
While DMEPA’s review process relies on complex computer evaluations, they also incorporate the human element into their studies. The reviewers undertake what’s known as name simulation studies, in which FDA-affiliated nurses, pharmacists, and doctors are presented with both written and oral prescriptions for the new drug and asked to interpret them. If the drug name is found to be an unacceptable source of confusion at any stage in the review process, the name is denied and a substitute chosen, which is what happened with the switch from Qnexa to Qsymia.
In that instance, Vivus representatives have confirmed that Qnexa’s similarity to existing drug names is what caused the problem. Reviewing the list of FDA-approved drugs, there are three medications that might well have caused problems for Qnexa. There’s Qnasl, Teva Respiratory’s spray for nasal allergies. And while it might seem unlikely that an oral medication would be mixed up with a nasal spray – unlikely, but unfortunately far from impossible – there’s also Qutenza, NeurogesX’s shingles medication, and the rhyming Ranexa, which is an angina medication from Gilead. Qsymia, on the other hand, is far more unusual. While it shares the “sym” sound with Simponi, Simulect, and indeed Amylin’s diabetes medication Symlin, the initial “Q” – already a rarity in English, as we’ll discuss in a moment – sets it apart from any of these. This doesn’t mean Qsymia will never be involved in a name mix-up, but the odds certainly appear much lower than they would have been for Qnexa.
While the FDA allows drug companies to devise their own potential trade names and then evaluates their safety, the USANC generally devises its own generic names. These names are built around “stems”, which communicate information about the medication’s drug class or how it works. Among diabetes medications, all DPP-4 inhibitors end in “gliptin”, all SGLT-2 inhibitors end in “gliflozin”, and all TZDs end in “glitazone” – even metformin, now the sole member of the biguanide class, once had sibling medications called buformin and phenformin, both long since withdrawn for safety reasons. It’s not a perfect system; the preferred stem for GLP-1 agonists is “glutide”, but Amylin derives Byetta and Bydureon from exenatide. And while this is certainly a logical approach to naming drugs, it does carry its own safety risks, as drugs with similar functions become even more likely to be confused when they share so many letters. For instance, since all sulfonylureas have generic names that begin either “gli” or “gly”, the ISMP has recommended capitalizing other parts of the generic name to more easily distinguish between them, turning the visually similar glipizide and glyburide into the far more distinct glipiZIDE and glyBURIDE.
The Stories Drug Names Tell
It’s also worth considering just what companies hope to achieve when they choose their proposed drug name. A drug’s name is its most powerful marketing tool in terms of reaching potential users, and so drug companies want names that evoke positive ideas and feelings. “Evoke” is the key word there, as the FDA specifically forbids any names that either “misleadingly imply unique effectiveness or composition” or “contribute to overstatement of product efficacy, minimization of risk, broadening of product indications, or making of unsubstantiated superiority claims.” A weight management drug like Qsymia likely could not have been called something like Qthinna, since the effective incorporation of “thinner” into the name would likely have been seen as overstating the product’s effectiveness as a weight loss drug.
 Looking at exotic product names like Qsymia, Belviq, and non-diabetes medications like Xalkori, Zyban, and Vfend, it’s easy to think that we’re not reading English at all and instead some weird alien language! We looked at a list of 842 FDA-approved medications (excluding duplicate names), and then analyzed how often each appeared in these drug names compared to their normal distribution in the English language. For instance, “h” accounts for about 6% and “w” for about 2.5% of the letters in all English words, but these two are barely used at all in drug names, at 0.78% and 0.18% respectively. This is likely due to pronunciation difficulties with these particular letters in other languages, which is why they also are typically avoided when crafting generic names.
Looking at exotic product names like Qsymia, Belviq, and non-diabetes medications like Xalkori, Zyban, and Vfend, it’s easy to think that we’re not reading English at all and instead some weird alien language! We looked at a list of 842 FDA-approved medications (excluding duplicate names), and then analyzed how often each appeared in these drug names compared to their normal distribution in the English language. For instance, “h” accounts for about 6% and “w” for about 2.5% of the letters in all English words, but these two are barely used at all in drug names, at 0.78% and 0.18% respectively. This is likely due to pronunciation difficulties with these particular letters in other languages, which is why they also are typically avoided when crafting generic names.
Some of the motivation behind this is to create words that sound futuristic or high-tech, hence the frequent use of otherwise uncommon letters like X, Z, and Q, with the latter often found without its normal partner U. The search for unusual-sounding words is also a byproduct of the need to secure trademarks for the medication name – pretty much every familiar word or even familiar-sounding combination of letters has been trademarked by now, which means drug companies need to stretch linguistics boundaries to find words they can actually legally use. The Internet also plays a part here, as a highly unusual name like Qsymia or Belviq is more likely to turn up first in search engines than a less original word.
Taken together, all these factors tell the story of how drugs get their name and what story a drug’s name ultimately tells to you. It may just be a random collection of letters – at times, what seems like an exceedingly random collection of letters – but those letters are intended to inspire hope and confidence and intended by the FDA and USANC to keep you safe from a serious error.







