Type 1 Diabetes: It’s Time for Population-Wide Screening
By April Hopcroft
 Experts are advocating for universal screening for type 1 diabetes. With the availability of Tzield and other medications on the horizon, there's a stronger push for screening earlier in life.
Experts are advocating for universal screening for type 1 diabetes. With the availability of Tzield and other medications on the horizon, there's a stronger push for screening earlier in life.
While type 1 diabetes is often thought of as a genetic condition that runs in certain families, the reality is that at least 85% of people who are newly diagnosed do not have a family history of diabetes.
A considerable proportion of these people find out they have diabetes through diabetic ketoacidosis (DKA), a condition of severe high blood sugar that can lead to coma or death if untreated.
With the rising incidence of type 1 diabetes, especially among youth and teens, better screening is needed to identify who will develop the condition. Early identification allows for proactive treatment and management, which reduces the risk of complications like DKA and heart, kidney, and eye disease.
Experts like Dr. Emily Sims, a pediatric endocrinologist at Riley Hospital for Children in Indiana, argue in favor of general population screening for type 1 diabetes. Sims cited the ability to effectively identify presymptomatic type 1 diabetes and delay the onset of diabetes using medications like Tzield (teplizumab) as key reasons for universal screening.
Testing for antibodies can identify type 1 diabetes
To implement population screening, there must be a reliable method to identify people at risk of developing type 1 diabetes.
Sims said that researchers and health care providers can identify presymptomatic type 1 diabetes in family members and the general population through antibodies; a large body of evidence shows that people with two or more islet autoantibodies (antibodies to the islet cells that produce insulin) have nearly a 100% chance of developing type 1 diabetes in their lifetime.
Testing for autoantibodies can be completed at home through the TrialNet clinical trial program, or at a doctor’s office or lab. For instance, JDRF’s T1Detect program provides at-home testing for $55, with lower-cost options for people in financial need.
Reviewing the stages of type 1 diabetes, Sims emphasized that the process of developing the condition takes place over years. As a result, there is ample time to intervene in the early stages.
-
Stage 1: At the beginning, people have at least two islet autoantibodies with normal blood sugar. Children who reach stage 1 have a 44% and 70% chance of developing diabetes in the next five and 10 years, respectively.
-
Stage 2: This is when people begin to show signs of abnormal blood sugar. There is a 75% risk of progressing to type 1 diabetes in the next five years in stage 2.
-
Stage 3: During stage 3, people are diagnosed with clinical type 1 diabetes and begin insulin treatment.
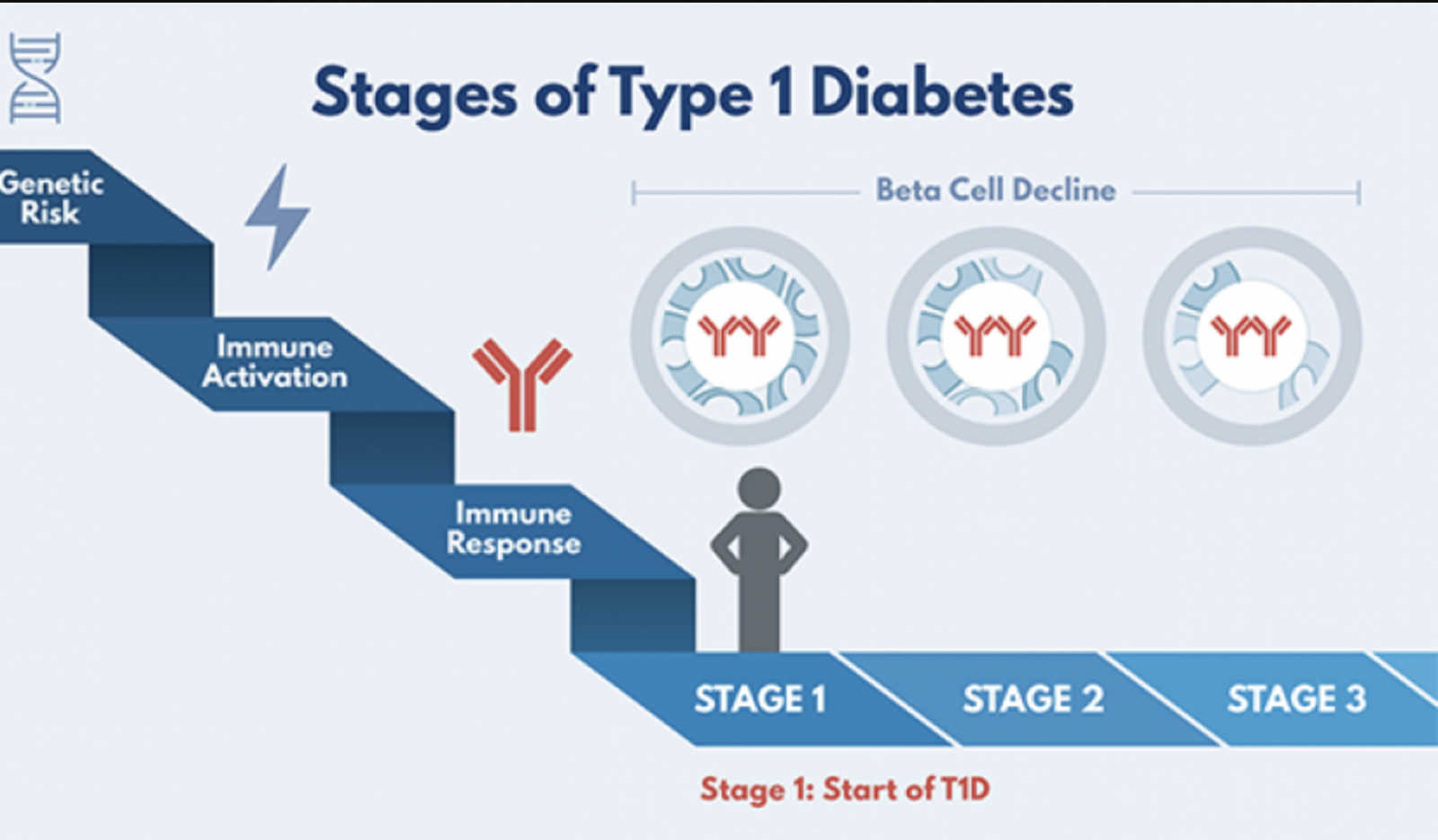
Photo credit: TrialNet
Early screening prevents complications and provides a safe landing for diagnosis
Sims discussed the potential to reduce rates of diabetic ketoacidosis at the onset of type 1 diabetes as a major reason for population screening.
DKA not only results in considerable costs for patients and the healthcare system, but it also has long-term consequences for brain function and glycemic control. Research shows that early screening and diabetes education lead to dramatic reductions in DKA rates.
That is, when caregivers know the signs of DKA, they can watch out for symptoms and seek medical attention right away. For instance, children in the TEDDY study who underwent screening had a DKA rate of 13%, compared to an expected rate of 24-55% without screening. Overall, Sims said education and monitoring reduces DKA from an expected rate of 25-50% to about 3-15%.
Beyond lowering the risk for DKA, identifying and monitoring people with presymptomatic type 1 diabetes allows for early education regarding all aspects of diabetes care.
Health care providers can slowly introduce different parts of diabetes management, rather than overloading a newly diagnosed patient with information all at once. For instance, it may be helpful to start with the concept of carb counting, followed by glucose monitoring, and then insulin treatment.
In this way, Sims said early identification allows for a “safe landing” and smooth progression into the diagnosis of clinical type 1 diabetes. Screening gives people time to consider questions, preferences, and goals for their eventual diagnosis.
Changing the course of type 1 diabetes
Tzield made history in November 2022, becoming the first FDA-approved medication to delay the onset of type 1 diabetes.
While the main clinical trial found that teplizumab delayed diabetes by an average of two years, follow-up data showed that the drug can delay type 1 diabetes by up to five years. Five years is a big difference – that could mean postponing a diagnosis of diabetes from kindergarten to fourth grade, or from ninth grade to college.
Prior to Tzield’s approval, the motivation to screen for type 1 diabetes was limited, since health care providers couldn’t offer any treatments to change the course of the condition. With the approval of Tzield, the conversation has changed. People who know they have two or more autoantibodies now have an option to halt the development of diabetes.
Indeed, the 2024 American Diabetes Association (ADA) Standards of Care recommend more intensive monitoring for the progression of preclinical type 1 diabetes. The Standards of Care also recommend using Tzield to delay the onset of diabetes in people at least 8 years old with stage 2 type 1 diabetes.
Looking ahead, there are several other drugs in development to delay or reverse type 1 diabetes:
-
Verapamil: This low-cost blood pressure drug has been shown to preserve insulin-producing cells in youth recently diagnosed with type 1 diabetes.
-
BMF-219: Known as an oral menin inhibitor, this drug is about to enter clinical trials for type 1 diabetes after yielding promising A1C reductions in participants with type 2 diabetes. Menin inhibitors have been shown to promote the recovery and growth of beta cells in animal studies.
-
VX-880: A stem-cell derived beta cell therapy, VX-880 led to significant improvements in A1C and time in range, in addition to reducing insulin needs in small studies of people with type 1 diabetes.
Advantages and disadvantages of general population screening
As research on screening for type 1 diabetes continues to evolve, different strategies have been proposed for population screening:
-
Genetic screening could help identify people at the highest risk and may lead to cost savings by screening fewer people overall. However, genetic screening involves a conversation to explain genetic risk and follow-up appointments to re-screen throughout one’s life.
-
Autoantibody screening for the general population allows for screening and diagnosis at the same time. However, researchers haven’t determined optimal ages for screening that are early and frequent enough to reduce DKA while still being feasible for the healthcare system.
Sims noted that either approach could work.
“What we need to do is start a lot of programs and scale them to understand how they’re working in different environments and healthcare settings,” she said.
Specifically, more studies on screening are needed in underrepresented populations as well as in adult-onset type 1 diabetes.
“It’s time for a paradigm shift in the way we diagnose and screen for type 1 diabetes,” Sims said. “General population screening is really the step to help the most at-risk people.”
Learn more about screening and prevention for type 1 diabetes:







