Tandem’s Control-IQ Hybrid Closed Loop Study in Children
By Eliza Skoler
 By Eliza Skoler
By Eliza Skoler
This currently recruiting study will support FDA submission of Tandem’s Control-IQ hybrid closed loop for children six years of age or older
Clinical Trials Identifier: NCT03844789
Trial Name: The International Diabetes Closed Loop (iDCL) Trial: Clinical Acceptance of the Artificial Pancreas in Pediatrics: A Study of t:Slim X2 With Control-IQ
Diabetes Type: Type 1 diabetes
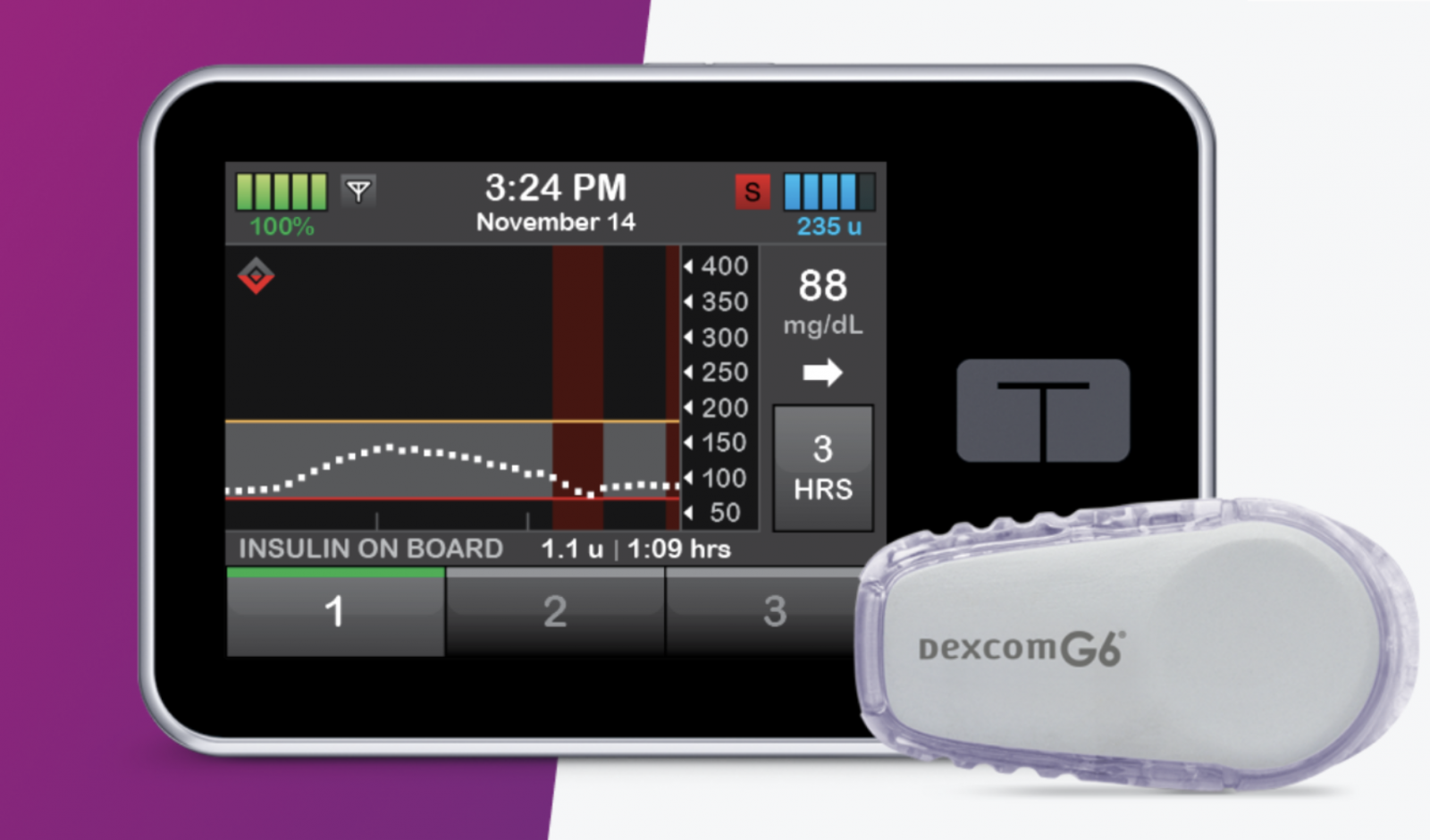
What the trial is testing: This four-month study will test use of Tandem’s Control-IQ hybrid closed loop system in children with type 1 diabetes ranging from 6-13 years old. Control-IQ is an automated insulin delivery (AID) system that combines the t:slim X2 insulin pump, the Dexcom G6 continuous glucose monitor (CGM), and a built-in Control-IQ algorithm that automatically adjusts basal insulin and delivers correction boluses for high blood sugars. Control-IQ aims to maximize time-in-range (70-180 mg/dl).
What the trial is measuring: This study will measure time-in-range (70-180 mg/dl) based on CGM readings, as well as time spent in high and low blood sugar ranges.
Why is this new/important? The Control-IQ system has already been submitted for FDA approval for people 14 years and older. There has been a lot of excitement around the impressive results of Control-IQ to improve time-in-range and lower A1C in people ages 14 and older. Results from this new study will be submitted to the FDA to expand the age range of people approved to use the product. Control-IQ is the first closed loop system with automatic correction boluses for high blood sugars and no fingerstick calibration needed for the CGM. Children tend to have more variable blood sugars than adults, and automated insulin delivery has shown impressive outcomes in younger age groups – improving time-in-range (especially overnight), reducing extreme blood glucose values, improving A1C, reducing diabetes burden, and giving parents more peace of mind. Medtronic’s MiniMed 670G hybrid closed loop is currently approved in the US for seven years and up.
When will Control-IQ launch? If approved by the FDA, Control-IQ is expected to launch in the US for people 14 years and older at some point in the last three months of 2019. It will be launched as a free software update for current Tandem t:slim X2 pump users. Tandem aims to use results from this trial to make Control-IQ available to children before next summer.
Trial Length: Four months, plus an optional three-month extension phase
Trial Locations:
-
California: Stanford (Stanford University)
-
Colorado: Aurora (Barbara Davis Center)
-
Connecticut: New Haven (Yale University)
-
Virginia: Charlottesville (UVA)
Click here for contact details.
Do you qualify? Eligibility criteria for the study include:
-
Six years to 13 years
-
Diagnosed with type 1 diabetes for at least one year and using insulin for at least six months
-
Living with a parent or guardian who knows what to do in case of severe hypoglycemia, and who will participate in all training sessions for the study
-
Willing to switch to Humalog or Novolog if not using already, and to use no other insulin besides Humalog or Novolog during the study (when assigned to use the t:slim X2 pump)
-
Cannot use any non-insulin diabetes drug other than metformin – this means no use of Symlin, GLP-1 agonists like Victoza and Trulicity, SGLT-2 inhibitors like Jardiance, Invokana, and Farxiga, and sulfonylureas
-
See full list of criteria here
Where to get more information:
Contact your closest site via the information listed under trial locations. The head of the study, Dr. Melissa Schoelwer, can also be contacted at mjs3pg@virginia.edu.
Learn more about Control-IQ:








