Pumping Insulin: New Technology May Spur Greater Acceptance
 by daniel trecroci and kelly close
by daniel trecroci and kelly close
The insulin pump has long been heralded as a revolutionary product in diabetes care – a brilliant delivery system that relieves patients of injections, a technology that can be programmed, manipulated, and tweaked to mimic a healthy pancreas. Pumps have their own evangelists who are mystified why any insulin-dependent patient would not use one, and in many ways their faith has been rewarded: the technology keeps getting better. Pumps are now more like powerful information-management systems than simple drug-delivery devices. They are small and discreet, they are being integrated with glucose meters and continuous glucose sensors, and they are central to the high-tech Holy Grail in diabetes management: the artificial pancreas. (Or, as some like to call it, the Artificial Pancreas.)
But for all their improvements, pumps have never become more than a niche product. In the US, only about 21 percent of type 1 patients use a pump, according to Diabetes Care, and very few insulin-dependent type 2 patients wear one. The numbers are even worse abroad: in the United Kingdom and in Denmark, for example, pumps are worn by about one to two percent of type 1 patients.
The product has never made greater inroads for several reasons. For one, many physicians are reluctant to prescribe them, let alone advocate for them. Clinicians must spend additional time to understand the pumps, additional time to train their patients, and additional time to handle the paperwork – but unbelievably, for the most part, they receive no extra compensation for their labor. What is that about?! Many patients themselves are not willing to invest the additional effort that pumps require. Money is also a factor. The pumps are expensive – about $6,000 for each device, excluding all the supplies – and some employer-sponsored health plans are cutting back on durable medical equipment coverage. No matter that pumps can improve our health dramatically over time.
Some experts blame the pump companies for not educating patients on why they should use the product and for not training their existing customers on how to take full advantage of its many features. Those are valid points.
Some observers contend that the pump companies don’t understand what they are promoting. We don’t go that far, but we definitely believe not all patients receive the right information about what pumps can do, so they are operating in the dark.
To increase the use of pump therapy (which in the medical literature is called “continuous subcutaneous insulin infusion”), patient training and reimbursement will have to improve. But so too will the technology. Like any sophisticated machine, pumps occasionally break down, and while the companies will replace them, there is nothing like a “dead pump” to throw a family vacation into turmoil. Temporary glitches require immediate troubleshooting, and serious problems can arise if the patient is unaware that – for whatever reason – the insulin is not reaching its destination. The true price of any pump is eternal vigilance that must start and end with the patient.
pump technology continues to improve
Continued improvement in pump technology may not guarantee a surge in sales, but pumps that are easier, smarter, and safer – and those that are integrated with meters and sensors – will stand a better chance. And the companies are spending their money accordingly.
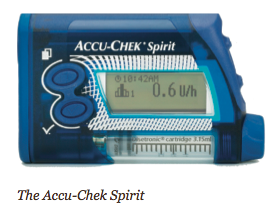
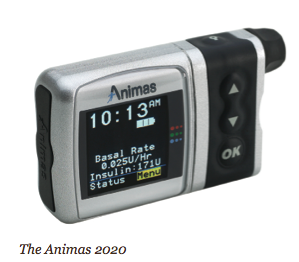
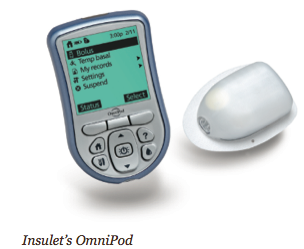
The MiniMed Paradigm REAL-Time System, for example, is the first to integrate an insulin pump with real-time continuous glucose monitoring. The new Animas 2020 has a flat-panel, high contrast color screen, the ability to store the previous 500 blood glucose values, and a food database. The Roche Accu-Chek Spirit has side-mounted tactile buttons for “no-look delivery” (you don’t have to withdraw the pump from your pocket, waistband, or bra). Deltec’s CozMore has a glucose meter attached to its back. And the one pump that does not rely on a tube – the OmniPod – consists of a lightweight device worn on the skin (like an infusion set), which delivers insulin according to signals from a handheld, wireless Personal Diabetes Manager.
how far we've come
It’s worth noting how far these pumps have come. The first models, in the 1960s, were mounted on backpacks. By the early 1980s, a pump could be attached to a belt, but it still weighed more than a pound, had flashing red lights, and was nicknamed “the blue brick” (though it was also reminiscent of a World War II-era walkie-talkie). Over the next two decades, the device became smaller, safer, more functional, more durable, and less uncomfortable.
Pumps today are about the size of a pager or even smaller, and we know size drives sales and uptake, so we can’t stress enough to the companies how critical it is to continue to reduce the size. Most pumps are composed of a reservoir that stores insulin, which is continuously delivered to the body through a tube that connects the reservoir to the cannula. The cannula is inserted underneath the skin at the site where insulin enters the body, and this “infusion set” is usually changed every three days. (Type 2 patients need more insulin so they may change the set closer to once every two days, while many type 1 patients can push the change to once every four days, which is possible with most pumps.) The battery-operated pump delivers the insulin according to how the patient programs it.
 how a healthy pancreas works
how a healthy pancreas works
Whatever its configuration, the pump’s aim is to replicate a healthy pancreas, which continuously secretes a low level of “background” insulin. But when the beta cells detect rising glucose levels, their insulin production goes into overdrive until glycemic order is restored. Imagine a graph with a flat production line interrupted by sharp spikes – that’s a healthy pancreas secreting background and mealtime insulin. People with diabetes try to recreate that graph through multiple daily injections of long- and short- acting insulins. This “basal-bolus therapy” is considered the gold standard for intensively managed patients, but an insulin pump is a more powerful, more flexible tool. It can be programmed to deliver a continuous amount of background, or basal, insulin, it can deliver a high-volume bolus dose before eating (typically, fast-acting insulin is used), a lower basal rate can be used during exercise, and “correction” doses can also be taken. “Pumps allow us to vary basal insulin levels to match the liver’s varying secretion of glucose through the day and night, and boluses delivered by the pump can be given with extreme specificity or delivered over a controlled period of time for slowly-digesting foods,” says Gary Scheiner, author of “Think Like A Pancreas,” in an interview with diaTribe.
compelling “smart” features become standard
 Some of the more compelling “smart” features on the pump have become standard, and “insulin on board” is one of the most important. When you give an injection, the insulin remains in the body for a number of hours, but you don’t know how much is still active and for how long. Pumps now come with software programs that make that information available. The feature deters patients from “stacking insulin” – or giving a correction bolus for a high blood sugar when the existing insulin (“insulin on board”) may return glucose levels to normal. “One of the complexities of pumping insulin is that people give multiple [boluses] through the day, and the current rapid insulins have a duration of action that is far longer than most people realize,” says John Walsh, co-author of “Pumping Insulin.” “A conservative estimate is that it lasts at least five hours. The average time between boluses is probably less than four hours. So there is a lot of overlapping of bolus activity.”
Some of the more compelling “smart” features on the pump have become standard, and “insulin on board” is one of the most important. When you give an injection, the insulin remains in the body for a number of hours, but you don’t know how much is still active and for how long. Pumps now come with software programs that make that information available. The feature deters patients from “stacking insulin” – or giving a correction bolus for a high blood sugar when the existing insulin (“insulin on board”) may return glucose levels to normal. “One of the complexities of pumping insulin is that people give multiple [boluses] through the day, and the current rapid insulins have a duration of action that is far longer than most people realize,” says John Walsh, co-author of “Pumping Insulin.” “A conservative estimate is that it lasts at least five hours. The average time between boluses is probably less than four hours. So there is a lot of overlapping of bolus activity.”
Several pumps will recommend how much insulin to deliver based on numerous criteria: your target range, your sensitivity to insulin, how many carbs you’re about to eat, and your current glucose level. The pumps have safety features to alert users when the insulin is not flowing properly or when the reservoir is low. They also have “multiple bolus options” – the “square-wave” and “dual-wave” boluses can spread the delivery of bolus insulin over time and are helpful when eating high-fat meals or when taking insulin with Symlin (See last issue’s Learning Curve for more information on this new drug for type 1 and type 2 patients who take insulin).
next generation: smaller and more integrated with other technology
What will the next generation of pumps look like? They will continue to become smaller, perhaps with more concentrated insulins in micro reservoirs. The tubeless, disposable OmniPod, which is easier to attach and has fewer “moving parts” than conventional pumps, appears to be a glimpse into the future: Other new pumps currently under development promise to be miniaturized devices taped directly onto the skin that deliver insulin subcutaneously. Like the OmniPod, other new pumps may be operated and programmed through remote control devices. Pumps will also become more “customizable” with personal alarms, reminders, and delivery options, and there will be a continued push to integrate with glucose meters and continuous glucose sensors. We also look forward to dual-chamber pumps that will allow us to infuse other drugs such as Symlin and perhaps Byetta (currently prescribed for type 2 patients).
better pumps are central to an artificial pancreas
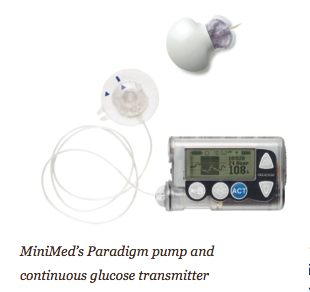
The ultimate prize is the artificial pancreas. In that scenario, a continuous sensor would signal to a pump how much insulin to deliver based on the current glucose level and its rate of change, and the pump would deliver the insulin without any human intervention. Some have called this closed-loop system a “technological” or a “mechanical” cure, or at least a way to ensure normal blood sugars regardless of how many pancakes you eat. The closest product to an artificial pancreas that is available is the MiniMed Paradigm REAL-Time Insulin Pump and Continuous Glucose Monitoring system. The sensor essentially “talks to” the Paradigm 522 or 722 pump, giving the user access to real-time glucose readings every five minutes and recommendations on boluses. It is still an open- loop system, because the patient delivers the insulin, and we believe open-loop systems will continue to improve and that, in fact, the power of the open loop may be underestimated. Getting to a true closed loop requires more than a more perfect sensor. It also requires the right algorithms and probably glucagon (what’s known as a counter-regulatory hormone), which in turn may require a dual-chamber pump.
Studies at Yale University have demonstrated the feasibility of a fully closed loop system, though they’re being done in the clinic and with significant monitoring. The trials are part of the JDRF’s (Juvenile Diabetes Research Foundation) Artificial Pancreas Project. The biggest hurdle right now is that the insulin, sent by pumps into subcutaneous fat, takes too long to take effect and then takes too long to stop working. This is true even for the fastest- acting insulin; and even if a more rapid-acting insulin becomes available, that might still be too slow. This is why, in addition to progress with new pumps, we are also closely following developments where faster-acting insulins, more concentrated insulins, and other valuable hormones could be infused, all with the goal of making a diabetic’s body more closely resemble that of a healthy person without diabetes.







