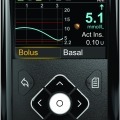
On June 11, Glooko and the Joslin Diabetes Center announced the release of their HypoMap software , a cool product that really should be able to identify and help...
Prevention
43 readers recommended
27 readers recommended
The Centers for Disease Control (CDC) has released its new data on the state of diabetes in the United States, providing an update on its 2011 National Diabetes Fact...
38 readers recommended
Twitter summary: Exciting positive vote for #liraglutide #emdac on possible new therapy for #obesity! Plus, our favorite open public hearing yet. Yesterday on September...
30 readers recommended
Why 2015 may be the “tipping point” for digital therapies in diabetes.
27 readers recommended
How a dramatic 23% increase in new diabetes cases impacts people living with diabetes nationwide.
31 readers recommended
Starting in 2012, the American Diabetes Association established the Pathway to Stop Diabetes initiative, a $1.6 million grant (paid over five years) to support the...
48 readers recommended
The latest on Medtronic’s drive to close the loop!
27 readers recommended
Can a drug long-used to treat gout be effective at preventing diabetic kidney disease?
30 readers recommended
How this “disease interception” strategy could transform our knowledge of type 1 diabetes.
41 readers recommended
Expansion of the use of Roche’s Lucentis a major step for patients.
41 readers recommended
How a single change at the FDA could lead to major improvements for patients and the healthcare system.
41 readers recommended
Leading ophthalmologist Dr. Ivan Suñer on what diabetic retinopathy is, how to prevent it, and what future treatments hold.
22 readers recommended
Praluent now approved for two specific patient populations. How this drug has the future potential to make a big impact on people with diabetes.
23 readers recommended
What does this controversial data mean for the type 2 diabetes epidemic?
42 readers recommended
Our top actionable tips for parents: straight from Friends for Life!
44 readers recommended
ADA, CDC, AMA, and the Ad Council just launched the first-ever national PSA campaign targeting prediabetes awareness and prevention - plus, three short video ads!
24 readers recommended
Diagnoses stabilizing, though 83% of 65+ year olds have diabetes or prediabetes.
29 readers recommended
Leaders in diabetes weigh in at The diaTribe Foundation’s second-annual event in Europe.
49 readers recommended
Gamechanging Xultophy (Tresiba + Victoza) also now submitted to the FDA.