App allows FreeStyle Libre sensor users to monitor blood sugar on their smartphone without a separate reader. Tools also available for sharing glucose data with...
Diabetes Device News
Diabetes Devices monitor and help to manage your blood sugar levels to try to stay within the target range, helping prevent complications of diabetes.
41 readers recommended
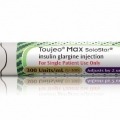
Toujeo Max SoloStar will launch later this year in the US. It holds 900 units of insulin and can dose up to 160 units in one injection, meaning fewer refills and...
30 readers recommended
Learn more about DiabetesMine D-Data highlights, implantable CGM Eversense used in adolescents, and Sugar.IQ real-world data
42 readers recommended
Will begin to launch in four European countries this year; wireless handheld controller with Bluetooth, 200-unit reservoir
30 readers recommended
Available for BCBS members in South Carolina and Arkansas, with Georgia coming soon; program includes an app, coaching, a connected meter, and unlimited supplies at no...
31 readers recommended
Low-cost education services can help you learn new skills to eat well, stay active, and monitor blood sugar levels
34 readers recommended
Read about combo drug Xultophy, Toujeo vs. Tresiba, diabetes apps, the do-it-yourself community, FreeStyle Libre real-world data, and more!
46 readers recommended
New dose adjustment recommendations for adults and children using Dexcom's G5 CGM based on trend arrows and insulin sensitivity
46 readers recommended
At the Lilly Diabetes Bloggers Summit, diaTribe got to hold a prototype of Lilly’s new, disk-like pump, which will integrate with Dexcom G6 CGM in a hybrid-closed loop...
30 readers recommended
Using time-in-range to assess risk for complications, Omnipod Horizon Closed Loop, Bihormonal closed loop (insulin + Pramlintide), SGLT-2 oral drug for type 1, and more!
46 readers recommended
FDA clearance and limited US launch expected in the second half of 2018. Dash adds improved user experience, Bluetooth, smartphone viewing, remote monitoring
44 readers recommended
Less intimidating insertion for pump users: peel tape, remove cover, one button to insert; available in Europe, with more countries coming later this year
44 readers recommended
New Dexcom G6-compatible Basal-IQ system prevents/reduces hypoglycemia based on CGM; US launch expected in August, including free software update for current users
51 readers recommended
Delays caused by sensor shortages largely resolved; MiniMed 670G now ships as soon as insurance is approved; a new assistance program to help with upfront costs
47 readers recommended
Dexcom G6 cleared by FDA with no fingerstick calibrations, 10-day wear time, approval for ages 2 years and older, no acetaminophen interference, and more
31 readers recommended
If you have a Fitbit, check out the diabetes communities in the app for tips and support from fellow people with diabetes
60 readers recommended
First implantable CGM available in the US! Eversense is expected to launch July 2018
33 readers recommended
The S-ICD is an FDA-approved device that can prevent sudden cardiac arrest; this trial is testing it in people with diabetes who’ve had prior heart attacks with...
49 readers recommended
Limited launch in May-July. No receiver! Smartphone-only display on Apple iOS devices. Plus, the new “personal diabetes assistant,” Sugar.IQ with Watson
25 readers recommended
ADA is around the corner, and there’s going to be exciting updates on diabetes drugs and therapy, devices, big picture questions, #beyondA1C, and advocacy; stay tuned!