Medtronic Recalls Minimed Paradigm and 530G Systems Due to Hypoglycemia Concerns Resulting from Minor Device Design Error
 Twitter summary: Medtronic recalls MiniMed products due to minor design error leading to accidental hypo – new design in the works to fix the issue
Twitter summary: Medtronic recalls MiniMed products due to minor design error leading to accidental hypo – new design in the works to fix the issue
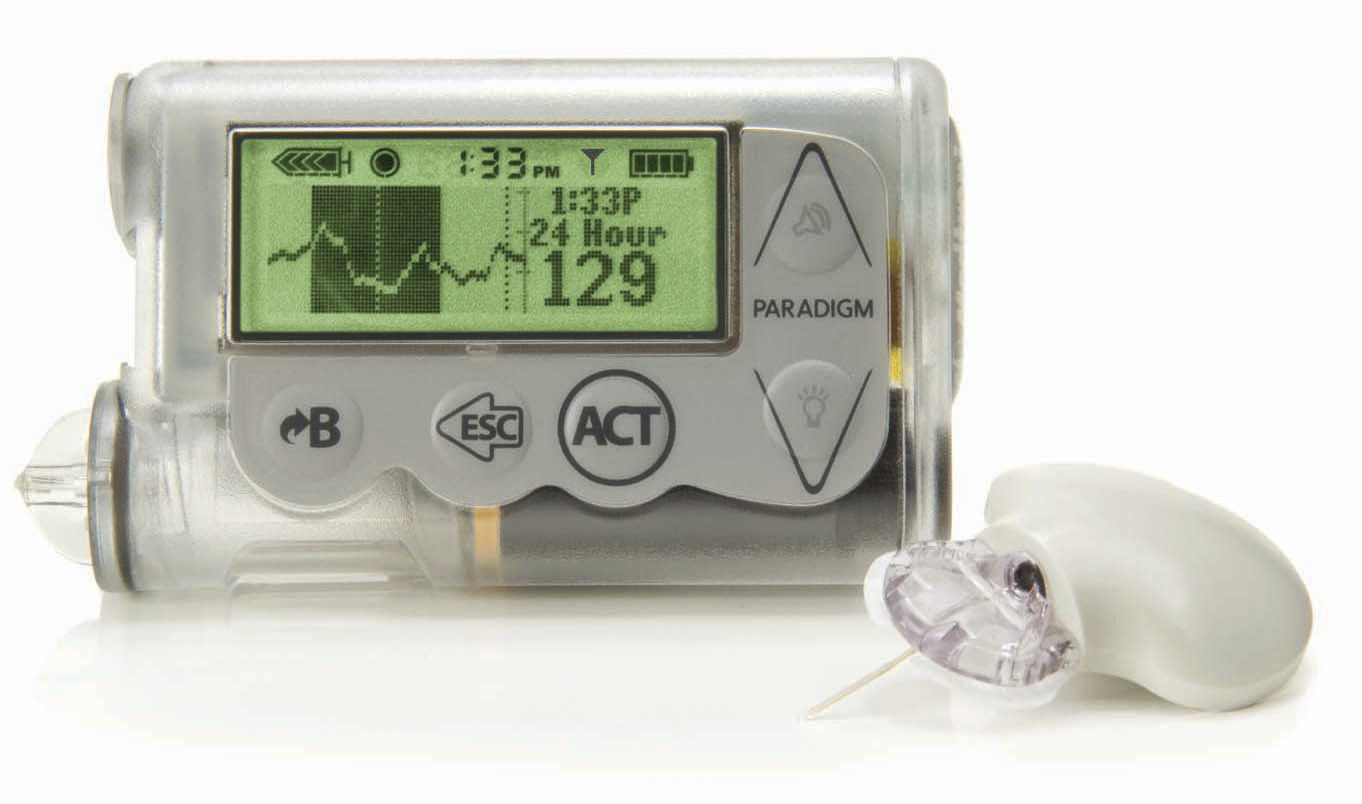
On August 22, the FDA posted a voluntary class II recall of Medtronic MiniMed Paradigm and 530G systems. There are three levels of FDA recalls (class I, II, and III), with a class II recall indicating an intermediate threat level, for those that can pose some health risks that are typically preventable and are not cause for major alarm. In this case, Medtronic mailed notices to customers earlier this year about the risk of an accidental overdose of insulin. Evidently, scrolling down past “zero” on the insulin dosing screen immediately scrolled to the maximum bolus dose of insulin, allowing users to accidentally give much more insulin than intended (e.g., hitting the down arrow once would scroll from 0.0 units to 10.0 units).
Reports of this serious error have fortunately been rare, with only one reported instance of severe hypoglycemia resulting in hospitalization. Medtronic already wrote its customers in March describing the issue and what safety precautions should be taken to minimize this hypoglycemia risk, and with this recall they plan to change the design such that the scrolling feature stops at zero. The FDA announcement said that 559,374 Paradigm and 530G systems had been recalled to date, impacting all Medtronic customers. Those who have Medtronic pumps do not need to return their pumps, but should read the safety information posted here. -AJW







