Join This Clinical Trial and Get Access to a Continuous Glucose Monitor
By Arvind Sommi
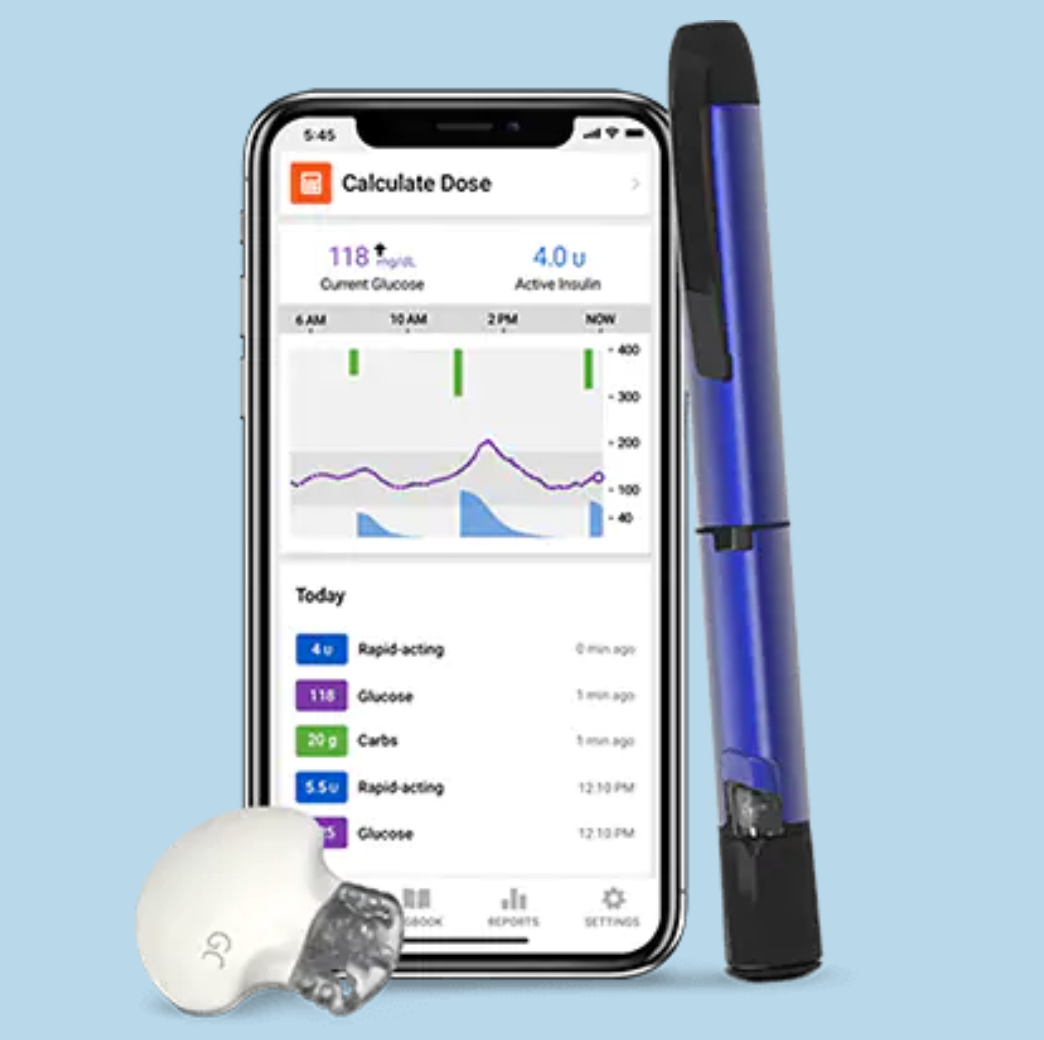
If you are currently taking three or more insulin injections per day, you may be eligible to participate in a clinical trial that provides you with the Guardian Connect continuous glucose monitor (CGM) system, InPen smart insulin pen, and smart insulin pen caps.
Clinical Trials Identifier: NCT04809285
Trial Name: Use of the Guardian Connect System With Smart Connected Devices
Diabetes Type: Type 1 and type 2
Trial Locations: This is a multicenter study with ten locations across the United States in Arkansas, California, Georgia, Tennessee, Texas, and Washington. For all study locations, click here.
What is the trial testing?
This data-collection study is looking for 500 participants to track a variety of health information for a minimum of 90 days to a maximum of nine months for subjects 18 years or older. Throughout this time, various applications will track the participants’ insulin delivery, sleep, physical activity, food intake, and medication (meal logging and medications will require manual entry). Additionally, participants have the option to record and upload their insulin injections, track their monthly menstrual cycle, and/or heart activity monitoring using the BodyGuardian MINI monitor.
The study will primarily measure participants’ Time in Range (TIR), defined as the percentage of time each person spends within the glucose range of 70-180 mg/dL. The study will also measure the percentage of time participants spend below range (less than 70 mg/dL) and above range (greater than 180 mg/dL).
Why is this new and important?
By volunteering to participate in this clinical trial, you will be provided with the Guardian Connect system (a real-time continuous glucose monitor (CGM)), InPen smart insulin pens, or insulin pens with smart caps. The CGM provides you with TIR data while the smart insulin pens keep track of how much insulin you have taken and can help you calculate dosing. Data collection studies like this one can help device companies improve their products – making them easier to use and helping researchers identify unmet needs.
In addition, this study is particularly noteworthy in that TIR is the primary outcome being measured. Greater adoption of TIR as a clinically meaningful endpoint can help improve the lives of people with diabetes. To learn more about Time in Range and why it is important, check out diaTribe’s Time in Range resources.
Trial Length: A minimum of 90 days and a maximum of nine months (depending on if you are included in phase 2 of the study).
Are you interested?
You may be eligible to participate in this study if you are:
-
Between two and 80 years of age
-
Have a clinical diagnosis of type 1 or type 2 diabetes for:
-
at least the last six months for people between two and six years of age
-
at least the last twelve months for people between seven and 80 years of age
-
-
Use multiple daily injections of insulin (defined as three or more insulin injections per day, one of which is a long-acting insulin injection), and is currently using or is willing and can afford to use insulin pen(s) and pen cartridge(s)
-
Currently using or is willing to use the Guardian Connect CGM system during the study
-
Not currently using an insulin pump
For More Information: To learn more, please contact Eileen Sneeden at 818-576-5203 or eileen.sneeden@medtronic.com.








