Glucagon without Needles? Locemia’s Intranasal Glucagon Powder for Severe Hypoglycemia
 Twitter summary: Locemia presents compelling data on intranasal glucagon at #ATTD2015 – could reach patients by late 2016, no needles necessary!
Twitter summary: Locemia presents compelling data on intranasal glucagon at #ATTD2015 – could reach patients by late 2016, no needles necessary!
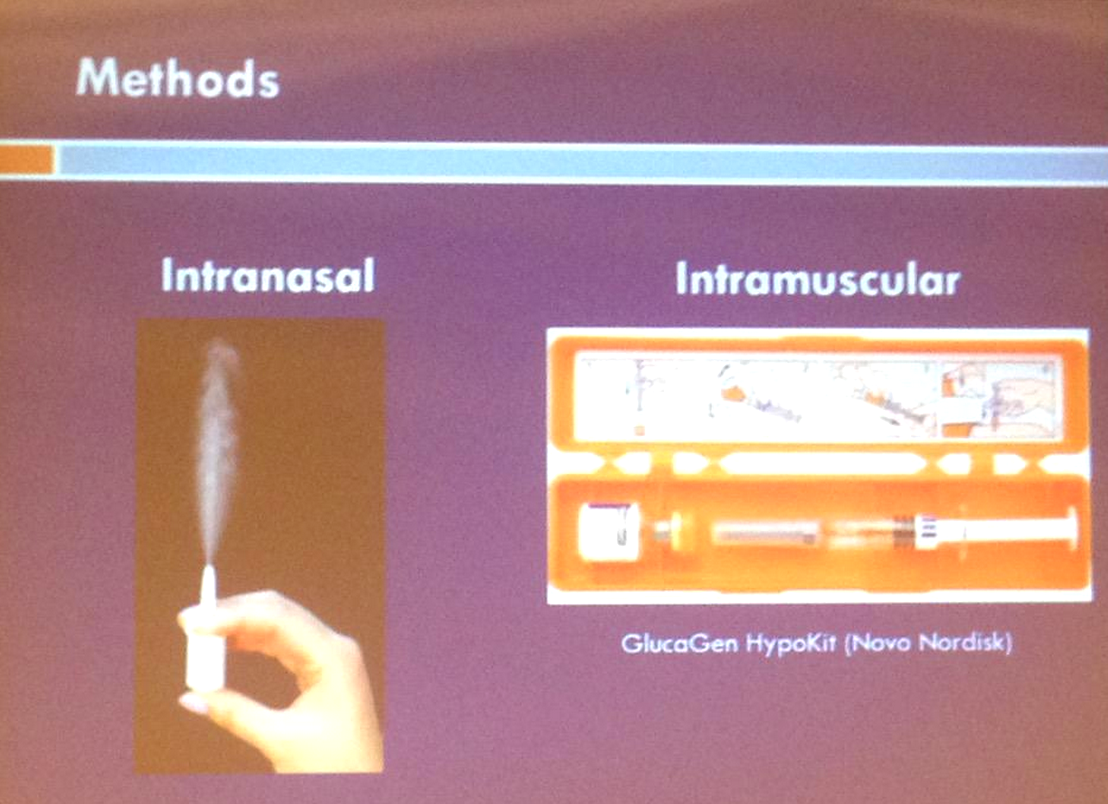
At the ATTD conference in Paris, Dr. Michael Rickels shared promising results from a phase 3 trial comparing Locemia’s intranasal (through the nose) glucagon powder to standard glucagon injection for the treatment of severe hypoglycemia (i.e., when someone with diabetes is unconscious/unresponsive or can’t safely ingest fast-acting carbohydrates). Locemia would be the first-ever intranasal, needle-free form of glucagon, a major improvement over intimidating emergency glucagon injection kits that require mixing powder/water (“reconstitution”) and delivering an injection.
Locemia’s novel intranasal design offers what looks like a quick and easy way to administer glucagon in case of a hypoglycemic emergency. Similar to a nasal spray for a cold, the one-time-use device has a small plunger on the bottom that, when pressed, releases glucagon powder up a nostril. No sniffing is required to absorb the glucagon, as the glucagon is absorbed in the nasal passages.
In the clinical trial, Locemia’s intranasal glucagon was just as effective as a standard glucagon injection in raising blood glucose. The intranasal glucagon was about five minutes slower in raising blood glucose vs. the injection. However, for caregivers who are called upon to treat severe hypoglycemia in the real world setting, we don’t think this five minute difference is important considering the no-needle advantage and push-of-a-button readiness of the intranasal glucagon compared to the time it takes to prepare and the potential errors associated with use of a traditional injectable glucagon kit. Pending success in the rest of its clinical trials, Locemia could potentially come to market with its intranasal glucagon powder as early as late 2016 or early 2017.
Locemia is not the only company working on improving glucagon delivery. Xeris is developing a ready-to-inject form of glucagon for severe hypoglycemia (an EpiPen-like device), a mini-dose pen for mild to moderate hypoglycemia (similar to an insulin pen), and a version of glucagon for use in the artificial pancreas. Biodel is also working on a simpler glucagon device. Both companies expect to submit their products to the FDA in late 2015 or 2016. We expect Locemia will be the first to market with a better glucagon, and we look forward to testing the device ourselves. –LE/AJW







