Glucagon through a Patch? A New Approach in Development to Address Severe Hypoglycemia
By Adam Brown
 Twitter Summary: Zosano completes recruitment of phase 2 trial of patch-based #glucagon delivery system for severe #hypoglycemia
Twitter Summary: Zosano completes recruitment of phase 2 trial of patch-based #glucagon delivery system for severe #hypoglycemia
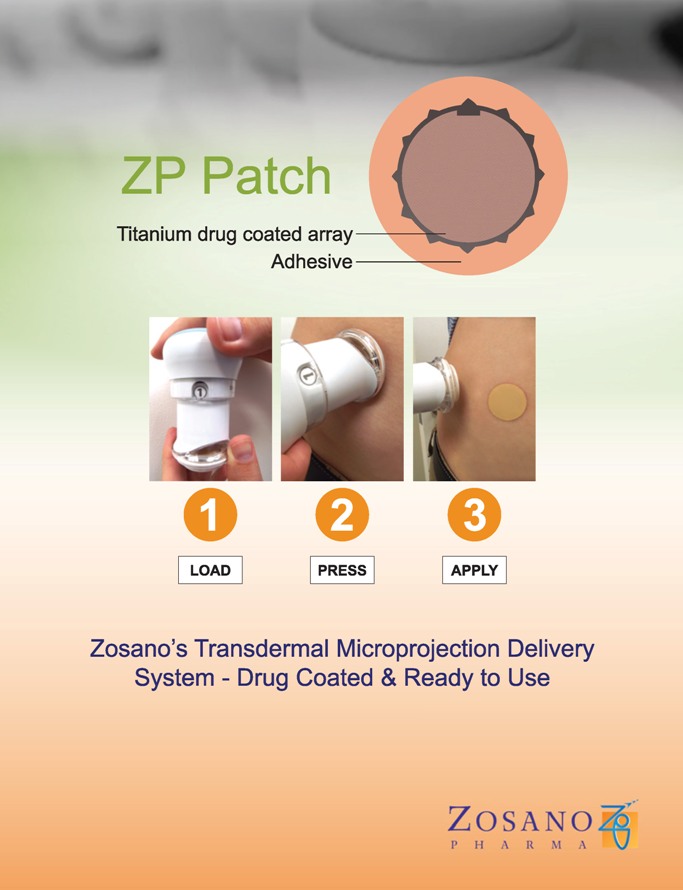
Zosano Pharma is making early progress with an innovative patch-based glucagon delivery system for severe hypoglycemia, recently announcing that it has completed enrollment for a phase 2 trial. The idea is to deliver glucagon through a coin-sized patch (about the size of a US quarter) that is applied to the skin. Early studies suggest the microneedle delivery can raise glucose just as fast as injected glucagon (starting in about 5-10 minutes), but in a design that is less scary and confusing than current glucagon kits. The Zosano system is still in early stages of research, meaning there is still much to prove on the product’s efficacy and the company’s ability to bring it to market.
 The ongoing phase 2 trial will be compare Zosano’s delivery system to standard needle-based glucagon injections. The researchers will give participants insulin to safely induce hypoglycemia, and then will observe the two glucagon-delivery methods’ impact on returning people to normal glucose levels, or “normoglycemia,” over a 30-minute observation period. This trial will further prove whether or not Zosano’s patch delivery system is as effective at raising glucose as standard needle injections. If all goes well, this trial could help set the stage for a phase 3 study that could potentially lead to its FDA approval.
The ongoing phase 2 trial will be compare Zosano’s delivery system to standard needle-based glucagon injections. The researchers will give participants insulin to safely induce hypoglycemia, and then will observe the two glucagon-delivery methods’ impact on returning people to normal glucose levels, or “normoglycemia,” over a 30-minute observation period. This trial will further prove whether or not Zosano’s patch delivery system is as effective at raising glucose as standard needle injections. If all goes well, this trial could help set the stage for a phase 3 study that could potentially lead to its FDA approval.
The design of this patch sounds very encouraging, particularly in high-stress times of hypoglycemia emergency. Compared to standard glucagon injection kits, which require mixing powder and water (“reconstitution”) and delivering an injection, this patch technology seems much more convenient and intuitive. Studies show very few people successfully deliver a full dose of glucagon using the current kits. Zosano also believes that this patch can be portable, without need for refrigeration.
In addition to Zosano, there are several other companies working on innovations in glucagon delivery.
-
Locemia is the furthest along, and has already completed phase 3 clinical trials of its intranasal glucagon delivery system (similar to a nasal spray for a cold). If all goes well, this could be available in late 2016 or early 2017, pending FDA approval.
-
Xeris is developing a ready-to-inject form of glucagon for severe hypoglycemia (an EpiPen-like device), a mini-dose pen for mild to moderate hypoglycemia (similar to an insulin pen), and a version of glucagon for use in the artificial pancreas. The Xeris pen for severe hypoglycemia is slated to enter a phase 3 pivotal trial this year, putting it chronologically behind Locemia and ahead of Zosano.
-
Biodel is also working on a simpler glucagon device that would auto-reconstitute the glucagon powder and water in three steps. The device is more complicated than the Xeris, Locemia, and Zosano approaches, but simpler than the current kits. A phase 3 trial is expected to start in late 2015 or early 2016.
-AJW/AB







