FreeStyle Libre Pro Submitted for FDA approval, Potentially Coming to the US in 2016
By Adam Brown
 Twitter Summary: @Abbott submitted FreeStyle Libre Pro to @US_FDA, with high likelihood of approval in 2016. How a blinded 14-day sensor can help patients + healthcare team
Twitter Summary: @Abbott submitted FreeStyle Libre Pro to @US_FDA, with high likelihood of approval in 2016. How a blinded 14-day sensor can help patients + healthcare team
What’s the latest scoop on Abbott’s FreeStyle Libre technology coming to the US? Abbott’s quarterly update call revealed that the company has submitted the professional version of FreeStyle Libre for regulatory approval in the United States. If all goes well, the product might be approved and launched in 2016.
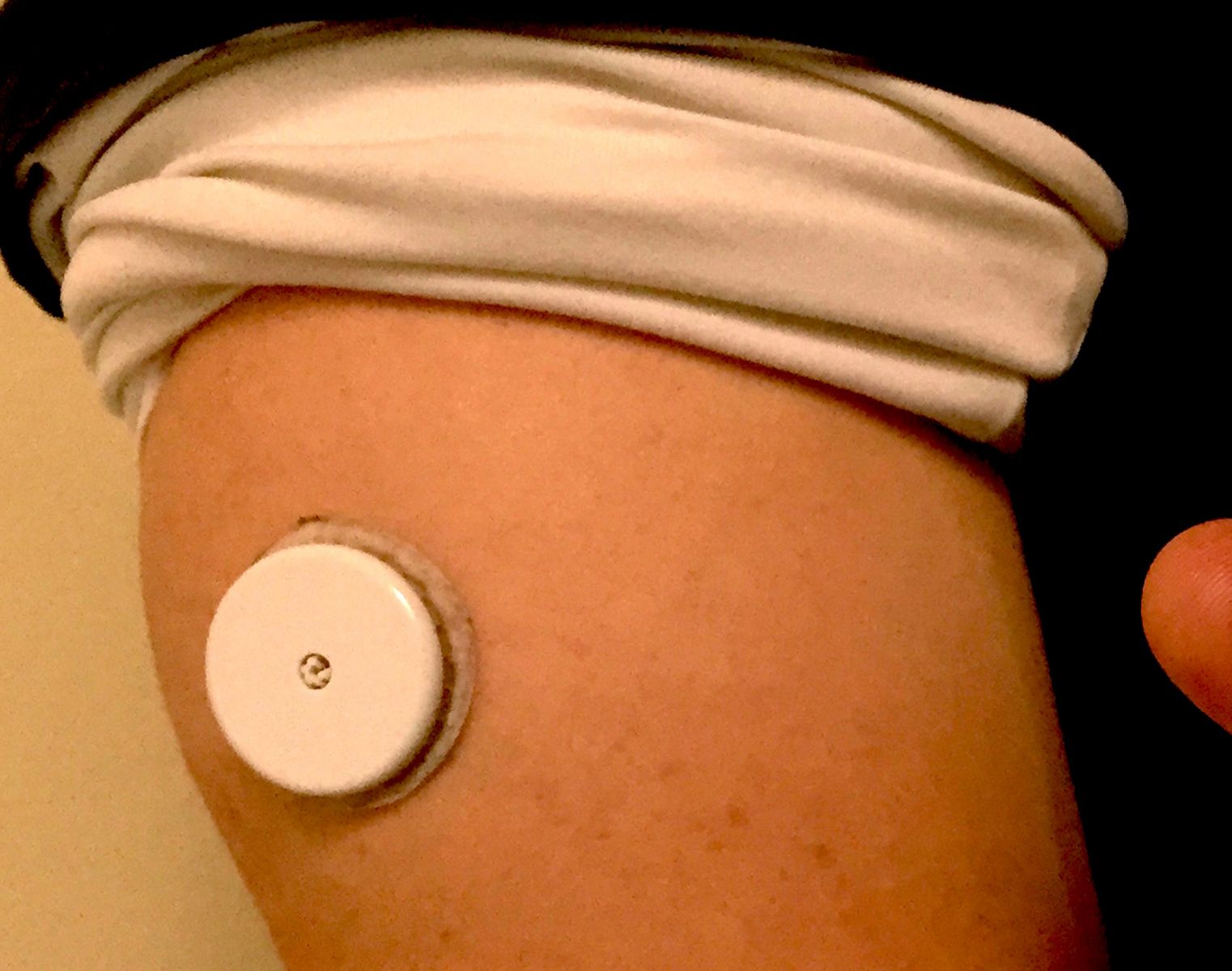
FreeStyle Libre Pro is a bit different from the patient version of FreeStyle Libre (available in Europe) that we tested in diaTribe in January. Libre Pro allows physicians to get continuous glucose data from patients over a two-week period. It consists of a small sensor (a bit larger than a US quarter dollar coin) worn on the arm. After applying Libre Pro in the doctor’s office, it is worn for two weeks, and the sensor automatically records glucose values every 15 minutes. Patients then return to the doctor’s office, where the sensor is downloaded. Abbott has done a really good job of making the glucose data download easy to interpret, meaning healthcare providers and patients can quickly grasp what is going well and what may need improvement.
Unlike the FreeStyle Libre system currently available in Europe, the Pro version does not give patients a reader device to look at glucose values in real time. While that seems like an obvious drawback, it’s a key design choice for a few reasons:
-
Many patients don’t want to wear a sensor all the time; wearing this product occasionally (i.e., twice a year) could offer many of the benefits of more continuous glucose monitoring (i.e., more comprehensive glucose data to change therapy), but without having to wear a sensor all the time.
-
Professional glucose monitoring systems like FreeStyle Libre Pro are generally reimbursed well by insurance, including Medicare. Medicare does not currently reimburse real-time CGM.
-
Many patients change their behavior in response to seeing the real-time data. A blinded sensor like FreeStyle Libre Pro makes it more likely patients will stick to their normal routine – allowing providers to get a more realistic view of a patient’s day to day management.
-
The FDA approval process for FreeStyle Libre Pro should be easier than the real-time version, since patients can’t make insulin dosing decisions off the blinded system.
Of course, we are huge fans of arming patients with real-time knowledge about their glucose, and we’re very excited about a real-time unblinded, commercial version of FreeStyle Libre coming to the US. For now, it’s not clear when that might happen, though we assume it will come after Libre Pro launches. The good news is that an FDA approval of FreeStyle Libre Pro might make the approval path for FreeStyle Libre easier. Our fingers are crossed! This US submission follows the Libre Pro’s launch in India earlier this year. –AB/NK







