Where Is Time in Range Headed?
By Natalie Sainz
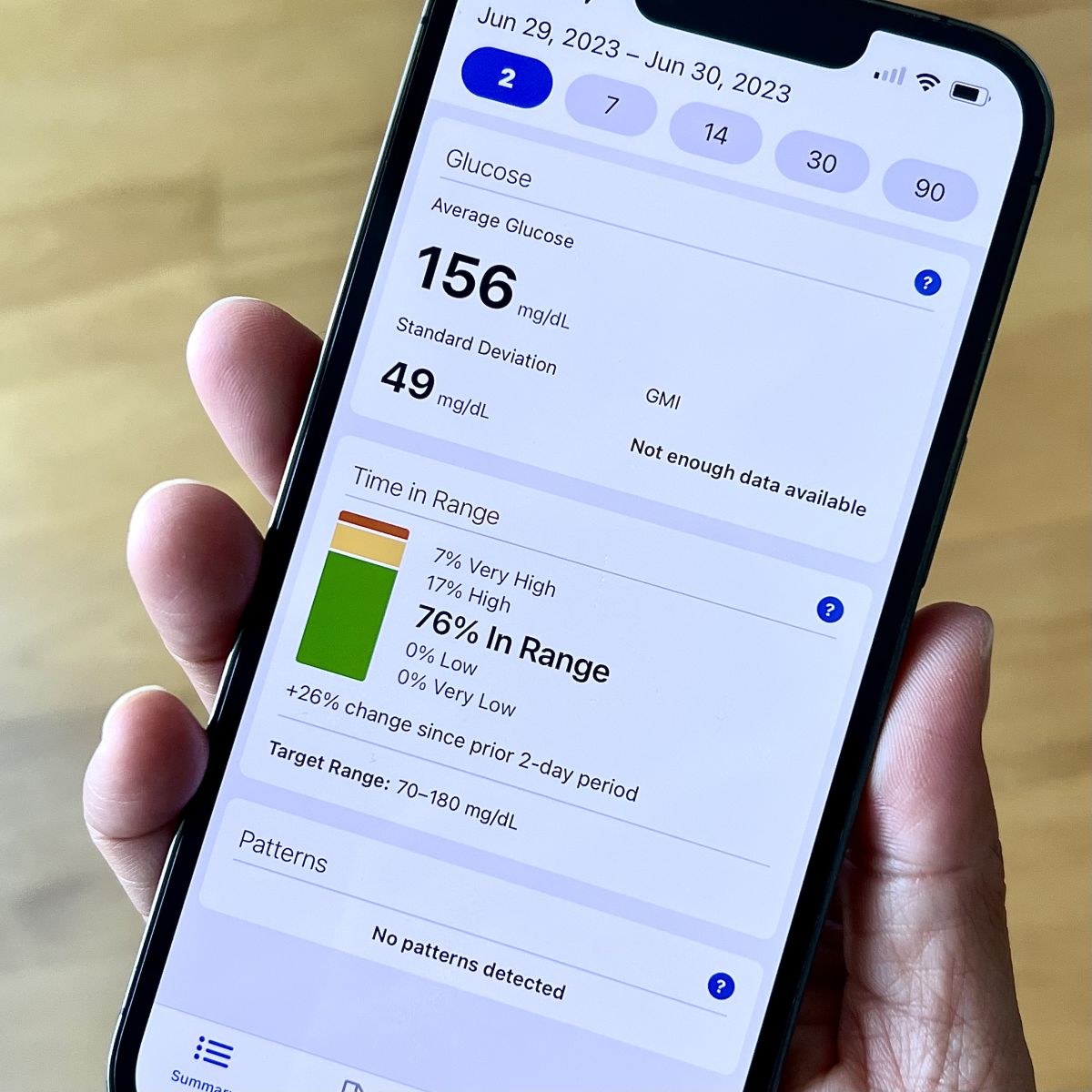 A lot has happened this year advancing time in range as a primary glucose metric in diabetes care globally. Though we aren’t there yet, a panel shared their thoughts on the progress and future of time in range.
A lot has happened this year advancing time in range as a primary glucose metric in diabetes care globally. Though we aren’t there yet, a panel shared their thoughts on the progress and future of time in range.
The Time in Range Coalition (TIRC), which was formed by diaTribe to establish time in range as an essential part of diabetes care, has made significant progress in making the measurement accessible to all people with diabetes.
At the ADA’s 83rd Scientific Sessions, a multi-perspective panel reviewed these advancements and shared their insights about what might be in store for time in range, including time in range (glucose 70-180 mg/dL), time below range (glucose less than 70 mg/dL), and time above range (glucose more than 180 mg/dL).
In clinical trials
Dr. Revital Nimri, a pediatric endocrinologist, strongly advocated for the use of time in range as a clinical endpoint as it enables people with diabetes and their healthcare teams to observe glucose fluctuations beyond A1C.
“Even those with the same A1C or average glucose levels can experience markedly different glycemic control,” said Nimri.
“The number of publications with TIR mentioned grew 12-fold between 2017-2021,” said Nimri, adding that the growing evidence points to the benefits of increasing time in range being associated with a reduced risk of long-term diabetes complications.
At the end of 2022, ATTD, diaTribe, and the TIRC published recommendations that set the standards of continuous glucose monitoring (CGM) use in clinical trials, which has enabled clinicians, scientists, and regulators to incorporate CGM measurements including time in range into their decision-making.
“Time in range and other CGM metrics should be used along with A1C and sometimes alone,” said Nimri.
At the regulatory level
Patrick Archdeacon, a medical officer with the U.S. Food and Drug Administration (FDA), spoke of the value that CGMs provide for people with type 1 and type 2 diabetes.
While he acknowledged the issues of only measuring A1C, Archdeacon said time in range alone should not be a primary endpoint for clinical trials because:
-
Time in range can provide correlated and complementary information to other metrics like A1C.
-
Not everyone's time in range is the same. For example, one person’s 70% can look different from another’s 70% as they might be spending significantly more time between 140-180 mg/dL rather than 70-120 mg/dL.
-
There are concerns about whether 70 mg/dL is clinically significant. Archdeacon said time below 54 mg/dL is an important glycemic measure (whether or not a person has symptoms) but was skeptical if 70 mg/dL is the correct cut-off for “in range” as 70-180 mg/dL “feels unjustified to treat all of that the same.”
-
Concerns about the accuracy of CGMs under 54 mg/dL (level 2 hypoglycemia)
The FDA recently published a draft guidance on diabetes efficacy endpoints for clinical trials that shares recommendations for companies to use when developing new therapies. Archdeacon encouraged everyone to submit comments about the document, which also includes recommendations about hypoglycemia.
In clinical care
Dr. Daniel DeSalvo, associate professor at Baylor College of Medicine, discussed the challenges and opportunities of promoting equitable access to CGMs and time in range-focused diabetes care.
He emphasized the importance of Medicaid coverage for CGMs, which varies by state and remains a significant barrier to widespread device use. DeSalvo said that expanding CGM access use would allow more patients to create personalized, adaptable goals for their diabetes care plan.
“Limited literacy and numeracy, insurance coverage, and data fatigue remain barriers to these new technologies,” said De Salvo.
Despite any challenges around time in range as a glucose metric, A1C alone is also not enough. “Diabetes care that relies on quarterly visits with A1C checks neglects the reality of life with diabetes that is continuous,” said De Salvo.
In patients
Kelly Close, founder of the diaTribe Foundation and Close Concerns, concluded the panel by describing how time in range has made her feel safer as someone who’s lived with type 1 diabetes for 33 years.
Close also presented research that showed how time in range leads to improved health outcomes. Retrospective CGM data from the Barbara Davis Center showed that every 5% decrease in time in range is associated with a 16% increased relative risk of developing diabetic retinopathy.
Additionally, about 28% of people with type 2 diabetes take sulfonylureas, which puts them at risk for hypoglycemia. CGM use in these individuals could help identify and reduce their time below range.
Close also shared her “CGM wish list,” which included that:
-
All people are asked if they want CGM and how they want their time in range to look.
-
Clinicians talk about time in range, as well as time below and above range.
-
CGMs are used in clinical trials.
-
Prediabetes screening is implemented.
-
CGMs are offered to those transitioning to insulin.
“We want to feel safer, be safer, and we want our effort to yield results. We need every tool we can get,” said Close.








