Bydureon Dual-Chambered Pen Receives FDA Approval After a Year-Long Wait
By Kelly Close
On March 3, AstraZeneca announced FDA approval for the Bydureon dual-chambered pen (once-weekly exenatide) to improve glycemic control in people with type 2. The device is a pre-filled, single-use pen injector, which eliminates the need for patients to manually mix the drug before injection (“reconstitution”) and reduces some of the “hassle factor” of taking Bydureon. Before, users of Bydureon had to go through a more involved process to prepare and inject the drug. AstraZeneca plans to make the pen available in the US later this year, and it is still under regulatory review in Europe with a decision expected by the end of this year.
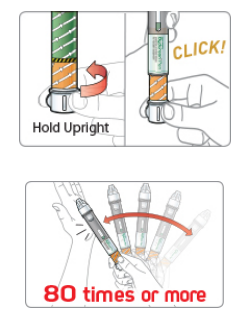
The new Bydureon pen preparation process does have a few hassles in this first generation and we look for more improvement over time. The pen will have to be refrigerated for storage and then must rest at room temperature for at least 15 minutes before use. The patient will attach the needle, then twist the base of the pen to mix the drug, and tap the pen firmly against the palm of their hand – the guide states that people may need to tap the pen “80 times or more” while rotating the pen until the solution is completely mixed. While this new process is a significant improvement from manual mixing, AstraZeneca is also working on a Bydureon suspension formula and auto-injector that will certainly increase convenience further.
Bydureon is a once-weekly GLP-1 agonist, which stimulates insulin production when blood sugar becomes too high. Please read our previous new now next articles about the approval of Bydureon, the action of GLP-1s, and our previous update about the Bydureon Pen for more information on GLP-1. We believe GLP-1 will continue to grow in use by patients due to its key advantages that differentiate it from insulin (weight loss or no weight gain, no association with hypoglycemia, and others). –NL/KC