ADA Makes Important Updates to Standards of Care, Adding Time-in-Range and More
By Jimmy McDermott
 By Rhea Teng and Jimmy McDermott
By Rhea Teng and Jimmy McDermott
New updates include time-in-range goals, heart health benefits of GLP-1 agonist Trulicity, and recommendation of GLP-1 agonist Victoza for children and teens with type 2 diabetes
The American Diabetes Association (ADA) recently made three important updates to its 2019 Standards of Medical Care – an influential document that provides healthcare professionals with recommendations and guidelines for diabetes care. These standards guide diabetes treatment and even influence insurers – they are a big deal. The new updates include:
1. The addition of time-in-range goals and metrics for people using continuous glucose monitors (CGM);
2. The addition of GLP-1 agonist Trulicity’s heart health benefits; and
3. The recommendation of GLP-1 agonist Victoza for children and teens with type 2 diabetes if their targets are not being met with metformin and/or basal insulin.
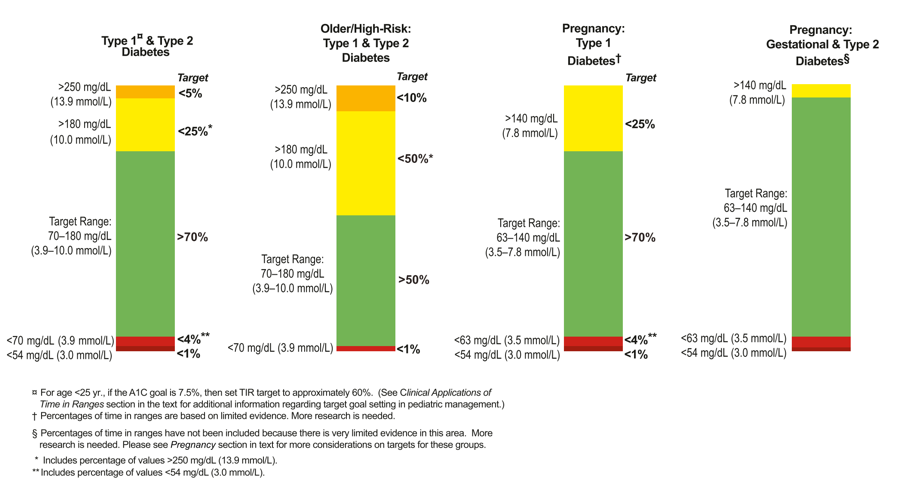
First, the Standards of Care now include time-in-range goals for people using CGM. This follows their publication at ADA and reflect important progress in the Beyond A1C and Time-in-Range movements. Previously, there was no agreement on what “range” to use, how much time should be spent in that range, and what a meaningful improvement would be. The range used and time-in-range goal should vary based on the person with diabetes (see below). No matter where someone is starting, every 5% improvement in time-in-range – e.g., going from 60% to 65% - is considered meaningful, as that’s one more hour per day. You can read the full time-in-range goals paper here.
-
For a person with type 1 or type 2 diabetes, the goal is to be in the target range (70-180 mg/dl) more than 70% of the time, with less than 4% spent in hypoglycemia (under 70 mg/dl). Some people with diabetes choose to use a tighter range of 70-140 mg/dl, though 70-180 is a good starting point for many.
-
For older or high-risk people with diabetes, the goal is greater than 50% time-in-range, with less than 1% in hypoglycemia.
-
For pregnant women with type 1, the goal is over 70% in the tighter range of 63-140 mg/dl. (Linked to lower risk of newborn complications.)
-
For pregnant women with gestational and type 2 diabetes, the goal is to spend the vast majority of the day in the tighter range of 63-140 mg/dl.
Second, new data on GLP-1 agonist Trulicity’s heart health benefits were added. At this year’s ADA, results from the REWIND trial showed that Trulicity, a once-weekly injectable GLP-1, resulted in a 12% reduced risk of combined non-fatal stroke, non-fatal heart attack, and heart-related death compared to placebo (a “nothing” pill). 2.4% of people taking Trulicity experienced these events, while 2.7 percent of people taking placebo experienced these events. Importantly, the Standards of Care update highlighted that Trulicity’s heart benefits were seen in both people with and without established heart disease. As such, REWIND offers the first evidence that GLP-1 agonists may prevent strokes and heart attacks in people without diagnosed heart disease.
Third, once-daily injectable GLP-1 agonist Victoza is now recommended for children ages 10-17 with type 2 diabetes. The ADA recommends that if blood sugar targets are not being met with metformin and/or basal insulin, then Victoza should be considered. This recommendation follows the FDA’s approval this past June for Victoza for children and teens with type 2 diabetes ages 10-17. A recently-published study showed that in this age group, compared to placebo, Victoza reduced A1C by an average of 1.3% (from a starting value of 7.8%) over the course of a year when added to metformin.
The full ADA Standards of Care are posted here. These updates are posted in the 6th (time-in-range), 9th and 10th (Trulicity), and 13th (Victoza) chapters. Please share this article with your own healthcare provider to make sure they are also informed!







